Agilis Robotics, developer of robotic instruments that support endoscopic surgery through natural orifices of the body, has recently completed the second round of live animal testing using its robot. Dr. Jason Y.K. Chan, the co-founder of Agilis Robotics, conducted the test with the firm’s second-generation device on a live pig specimen and successfully removed artificial tumors
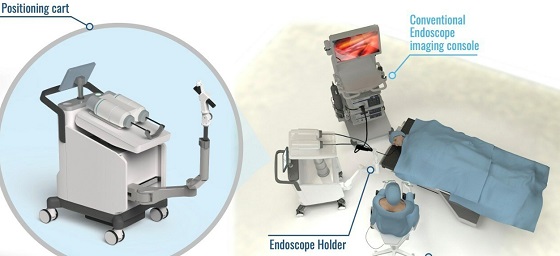
The Agilis Robotics system is a surgical robotic solution designed for endoscopic surgery. The system features a set of flexible surgical robotic instruments, a positioning cart and a surgeon’s control chair. The flexible robotic instruments, with a diameter of less than 2.8 mm, can grip and cut away tumor tissue to remove it in its entirety.
Drawing on the advice of surgeons in earlier experiments, the research team has made several technical improvements to the original system that increased the efficiency of the surgery. The second-generation prototype system used in this test includes several improvements over the original system. With a smaller footprint, the updated positioning cart is 50% smaller, making it highly compact and easily positioned in the operating room.
Safety
Second, the newly developed endoscope holder offers the surgeon great flexibility for positioning the endoscope and robotic instruments. During operation, the surgeon can move the endoscope with ease using just one hand in thanks to the holder’s sensor. The holder includes an automatic locking mechanism that prevents the unit from moving around freely, streamlining the surgeon’s work and improving the procedure’s safety.
The surgical tools have also been improved in this prototype iteration, allowing the surgeon to visualize the surgical field beyond the instruments more clearly and increase its load capacity to meet more demanding requirements. The electrosurgical knife instrument was also improved to enable bipolar diathermy, which substantially enhances tissue cutting effectiveness in bladder tumor resection. The most notable improvements are made to the controls and mechanical transmission in terms of precision and reduced control latency. This gives the surgeon a more intuitive and responsive control experience compared with manual operation. In addition to the controls, the team has also made substantial improvements to the system’s ergonomic design and training simulator.
Next, the company will test the system in animals for upper and lower gastrointestinal (GI) endoscopic submucosal dissection (ESD). This will be a major milestone for the company, demonstrating their potential within the sizeable market for GI diseases.